Doctors have hailed a “new era” of medicine after a study showed for the first time that a drug can slow the debilitating symptoms of Alzheimer’s.
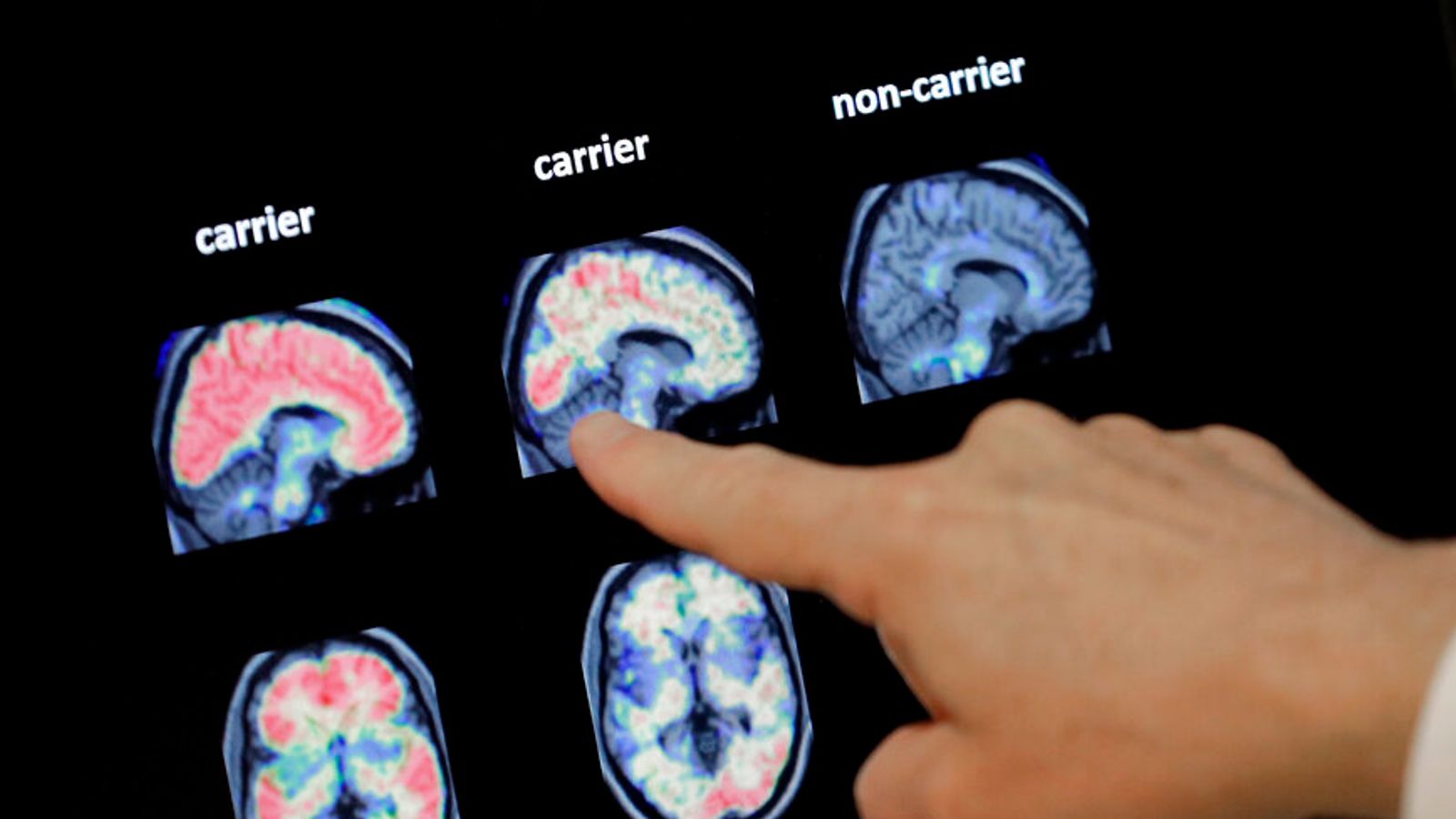
Results from the clinical trial also revealed that the drug lecanemab cleared clumps of a protein called amyloid – thought to be a key cause of the most common form of dementia – from patients’ brains.
The data, published at a conference in San Francisco, led to an outpouring of optimism from scientists, many of whom had spent decades trying to understand what leads to the disease and find a treatment.
Rob Howard, professor of old age psychiatry at University College London, said the results were “wonderful and hope-filled” – adding: “At long last we have gained some traction on this most terrible and feared disease and the years of research and investment have finally paid off.
“It feels momentous and historic. This will encourage real optimism that dementia can be beaten and one day even cured.”
The manufacturers of the drug released top-line results in a news release earlier in the autumn, but many doctors held back from celebrating until full results were released at the Clinical Trials on Alzheimer’s Disease conference.
They showed that lecanemab slowed the decline in memory and mental agility by 27% in patients with mild Alzheimer’s.
Government should provide insurance against dementia care costs, David Cameron says
‘Historic moment’ in Alzheimer’s treatment as trial drug found to slow cognitive decline
More than 70,000 in England living with early onset dementia, charity says
‘Doctors are optimistic’
Critically, the drug removed so much of the amyloid protein that the patients wouldn’t have had enough evidence of Alzheimer’s disease on their brain scans to actually qualify for entry to the trial.
The study strongly suggests that the drug only starts to have a clinical effect once amyloid is reduced to low levels in the brain.
Results after 12 months of treatment suggested it was ineffective – but after 18 months, the effect was significant.
Doctors are optimistic that continued treatment will lead to even better results.
Professor Nick Fox, director of the Dementia Research Centre at University College London, said: “It confirms a new era of disease modification for Alzheimer’s disease, an era that comes after more than 20 years of hard work by many, many people, with many disappointments along the way.”
Lecanemab is not a cure. But even slowing the progression of Alzheimer’s disease would be game changing, delaying the need for specialist care and allowing people to spend more time with their families.
However, the drug has side effects.
One in eight patients given lecanemab suffered brain swelling and other changes, probably as a result of removing the amyloid protein. But most only had evidence of problems on brain scans. Fewer than one in 30 had actual symptoms such as headaches or confusion.
Some patients had bleeding in the brain, though deaths were no higher in those receiving treatment than those given a dummy drug.
Nevertheless, it underlines the need for careful monitoring of those on treatment.
Prof Fox said: “Any risk is clearly important, but I believe that many of my patients would be very willing to take such a risk.
‘Massive challenge for the NHS’
Doctors warned that lecanemab will be a massive challenge for the NHS, not just because the drug is given through an intravenous infusion every two weeks.
Most Alzheimer’s patients are currently diagnosed when they have moderate symptoms – too late for treatment with lecanemab. And just 1% have their diagnosis confirmed by a brain scan or lumbar puncture, a biopsy of their spinal fluid.
Susan Kohlhaas, director of research at Alzheimer’s Research UK said: “It’s safe to say that the NHS is not ready for a new era of dementia treatment.
“We estimate that unless there are drastic changes in how people access specialist diagnostic tests for Alzheimer’s disease, only 2% of people eligible for drugs like lecanemab will be able to access them.”
Until now there have only been drugs that treated symptoms rather than the underlying cause. But if lecanemab is licensed for use on the NHS then delays in treatment will result in brain cells dying and the disease progressing.
Prof John Hardy, from the UK Dementia Research Institute in London said the drug had been “a long time coming”.
He added: “I truly believe it represents the beginning of the end.
“The first step is the hardest, and we now know exactly what we need to do to develop effective drugs. It’s exciting to think that future work will build on this, and we will soon have life-changing treatments to tackle this disease.”